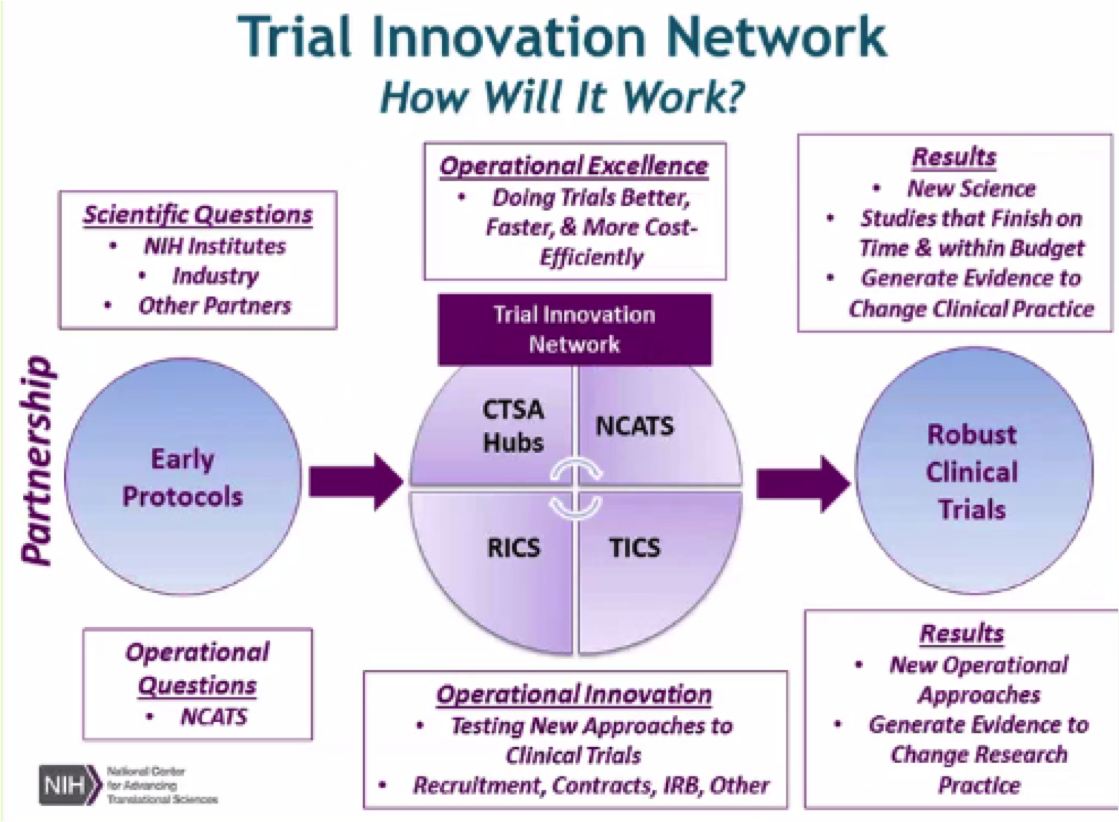
 The NCATS Trial Innovation Network (TIN) seeks to address critical roadblocks in multi-site clinical research and accelerate the translation of novel interventions into life-saving therapies. The TIN focuses on innovations in two key areas traditionally associated with creating administrative burdens for multisite studies: trial initiation and patient recruitment. It comprises three key organizational partners:
The NCATS Trial Innovation Network (TIN) seeks to address critical roadblocks in multi-site clinical research and accelerate the translation of novel interventions into life-saving therapies. The TIN focuses on innovations in two key areas traditionally associated with creating administrative burdens for multisite studies: trial initiation and patient recruitment. It comprises three key organizational partners:
-
Trial Innovation Centers (TICs): With a focus on operational excellence, operational innovation, and quality by design, the TICs are charged with coordinating and providing innovative, high quality operational support for TIN clinical trials.
-
Recruitment Innovation Center (RIC): An evidence-based center in innovative trial recruitment and retention methods, tools and strategies. The goal is to work with researchers to develop, test and share innovations in order to improve participant recruitment and retention and in doing so, improve health outcomes. It aims to raise national awareness of the importance of clinical trials and opportunities for the public to be involved.
-
CTSA Program Hubs: As key partners of the Trial Innovation Network, the CTSA Program Hubs play integral roles by:
- Encouraging faculty and investigators at their Institutions to generate ideas for trials and studies.
- Providing input before protocols are implemented.
- Recognizing the essential contributions and efforts of their local teams in executing multi-center clinical trials.
- Creating a culture in which key stakeholders play unique and important roles, and ultimately work together to build a national system to conduct clinical trials better, faster, and more cost-effectively.
The CCTS Hub has established a TIN Liaison team, which is responsible for coordinating with the national Trial Innovation Network. The TIN Liaison team helps provide reliable study activation, recruitment, retention, and data standards to most effectively facilitate clinical, translational, and comparative effectiveness multisite research focused on improving health outcomes. The team connects to activities among other multisite study stakeholders, including the Hub’s IRB, Office of Sponsored Programs, Clinical Trials Initiative as well as CCTS-led efforts in Informatics, Biostatistics, Workforce Development and Research Acceleration.
The team also interfaces with clinical and translational investigators through the CCTS Research Commons and helps connect the TIN to the capacities of our SHARe platform to expand the reach of studies to the populations in our region.
| ROLE | TEAM MEMBER |
|
| Medical Director |
Vivian A. Fonseca, MD, FRCP Chair, SHARe Advisory Board/ Medical Director, SHARe Asst. Dean for Clinical Research Chief, Endocrinology, Professor and Tullis-Tulane Alumni Chair—Diabetes Hub Liaison Team Scientific Lead |
|
| Program Co-Directors |
Christina Cenczyk, MSCJ , CCTS SHARe Administrative Director and Hub Liaison Team Operations Co-Lead Andrew Barton, MS, Hub Liaison Team Operations Co-Lead |
|
| Training, Contracting, Regulatory & Recruitment Director |
Meredith Fitz-Gerald, MSN Director, CCTS Clinical Research Support Enterprise (CReSt) Education & Community Partnerships |
