Currently used Food and Drug Administration-approved transplant drugs — with the addition of an also already FDA-approved complement inhibitor — are the optimal immunosuppression regimen for pig-to-human kidney transplants, according to a landmark discovery by University of Alabama at Birmingham investigators. The peer-reviewed research is published today in the Journal of Clinical Investigation.
“These findings establish the ideal immunosuppression regimen for pig-to-human kidney xenotransplantation, and it is a regimen that is already FDA-approved and that we routinely use for human-to-human allotransplantation,” said Jayme Locke, professor of surgery in UAB’s Marnix E. Heersink School of Medicine and lead author of the paper. “Our research found combining common immune-suppressing drugs with a complement inhibitor effectively managed the initial human immune response against transplanted pig kidneys.
“It’s a major departure from what’s been tried before in most non-human primate experiments and living human heart transplants, but it’s with a treatment regimen transplant doctors work with every day.”
The paper marks the third major peer-reviewed, published xenotransplant finding for UAB surgeon scientists since 2022. In each case, the team used the Parsons model, a pioneering pre-clinical human research model in a recipient experiencing brain death. The findings also continue to advance the science and promise of xenotransplantation as a therapy to potentially cure end-stage kidney disease — just as human-to-human allotransplantation can — and addresses the worldwide kidney organ shortage crisis. Currently, more than 800,000 people in the United States are living with kidney failure and 90,000 people are awaiting a kidney transplant.
Previously, in 2022, UAB’s research team announced the first peer-reviewed, published research in the American Journal of Transplantation which established that genetically modified pig kidneys were successfully transplanted into a recipient after brain death. That groundbreaking paper also recognized the Parsons model — developed at UAB in partnership with Legacy of Hope — could evaluate the safety and feasibility of pig-to-human kidney transplants without risk to a living human. It is named for transplant pioneer Jim Parsons, an organ donor whose family generously donated his body to advance xenotransplant kidney research.
Read all UAB peer-reviewed, published xenotransplantation breakthroughs at go.uab.edu/xenotransplant.
And just last summer, in a paper published in JAMA Surgery, UAB’s research team established for the first time that pig kidneys could provide life-sustaining kidney function in humans.
JCI paper details
The peer-reviewed findings in today’s JCI online publication examine the case series of the three decedent model xenotransplants Locke’s UAB team has conducted.
Researchers used standard immunosuppression in the first decedent model, but they did not use a complement inhibitor. While the biopsies of the pig kidneys were histologically normal in the first model after being transplanted on the first day, they began to show some signs of rejection the day after transplant.
 Click image to enlarge.
Click image to enlarge.
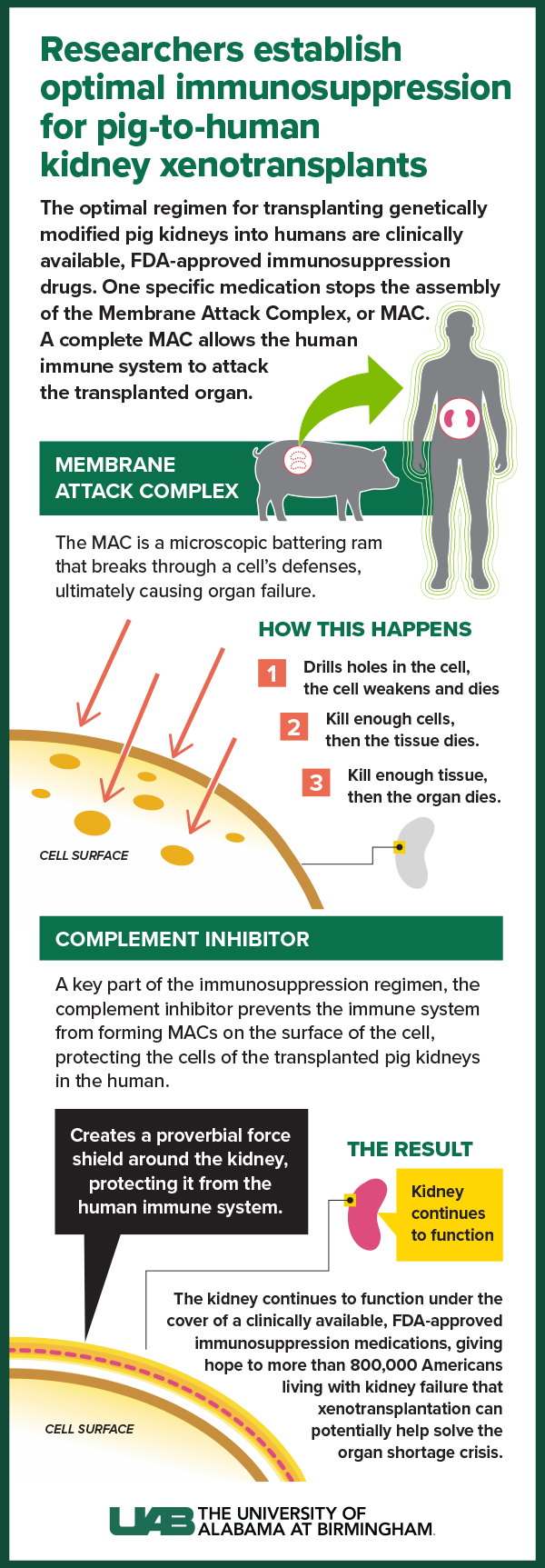
Graphic by: Jody PotterWith the second and third decedent model studies, UAB researchers used standard immunosuppression and added a complement inhibitor, which is already a common, FDA-approved regimen for patients with a rare kidney disease known as atypical hemolytic uremic syndrome or AHUS. The complement inhibitor prevented the immune system from forming a membrane attack complex — a microscopic battering ram that breaks through a cell’s defenses, ultimately causing organ failure. Instead, when the complement inhibitor was used, the transplanted pig kidneys did not reject and were able to provide life-sustaining function.
“The membrane attack complex essentially sets up like a little drill on the surface of a cell and it drills holes in the cell,” Locke said. “If you drill holes in the cell, it weakens and it dies. If you kill enough cells, then the tissue dies. If you kill enough tissue, then the organ dies. The complement inhibitor prevents the formation of this drill on the surface of the cell. It’s like creating a force shield around the kidney.
“This finding is great news. We now know that we have an FDA-approved immunosuppression regimen already available. It’s a regimen that we know the patient can tolerate, and it is a treatment transplant doctors are familiar with and already know how to use. This is another critical piece that I hope will soon lead to FDA clearance for a Phase I clinical trial in living humans.”
Study support
These studies are supported by biotechnology pioneer United Therapeutics Corporation, which awarded a grant to UAB to launch the innovative xenotransplantation program. Revivicor, Inc., a subsidiary of United Therapeutics, provided the genetically modified pigs that were the source of the investigational xenotransplant kidneys called UKidney™. The 10-gene edited pigs are raised in a secure, pathogen-free facility by UAB animal care experts and are regularly tested for porcine cytomegalovirus (pCMV) and porcine endogenous retroviruses (PERV), including PERV-C. The pigs used in these studies tested negative for PERV-C and pCMV.
“Our partners at Revivicor and United Therapeutics have been incredibly supportive of Dr. Locke and our entire UAB research team, and we are grateful for the progress we have made together,” said UAB Heersink School of Medicine Dean and Senior Vice President of Medicine Anupam Agarwal, M.D. “We hope these efforts continue to move us forward toward FDA clearance for a Phase I clinical trial in living humans and hopefully add a new, desperately needed solution to address an organ shortage crisis responsible for tens of thousands of preventable deaths each year.”
Legacy of Hope saves lives through organ and tissue donation and honors the legacy and generosity of donors. Learn more about donation or register to be an organ donor at legacyofhope.org.
This xenotransplantation study was led by the Division of Transplantation in the Department of Surgery, in close partnership with Legacy of Hope. Other key contributors to this work have included members of the Division of Infectious Diseases and the Division of Nephrology in the Department of Medicine; the Department of Anesthesiology and Perioperative Medicine; and the Department of Pathology. In addition to these specific contributors, the xenotransplantation program at UAB encompasses multiple departments and divisions of the Heersink School of Medicine and is also supported by numerous administrative and regulatory departments.
In addition to Locke, other UAB and Legacy of Hope authors on the JCI paper include Maggie Jones-Carr, M.D.; Huma Fatima, M.D.; Vineeta Kumar, M.D.; Douglas Anderson, M.D.; Julie Houp; Jackson Perry; Gavin Baker; Leigh McManus; Andrew Shunk; and Paige Porrett, M.D.
Disclosures: Locke reports consulting fees from Sanofi. Drs. Locke, Fatima, Anderson and Porrett report grant support from United Therapeutics and subsidiaries (Revivicor and Lung Biotechnology). United Therapeutics and its subsidiaries provided grant support for this research. The funder had no role in study design or conduct; data collection, management, analysis or interpretation; manuscript preparation or review; or in the decision to publish the manuscript.
