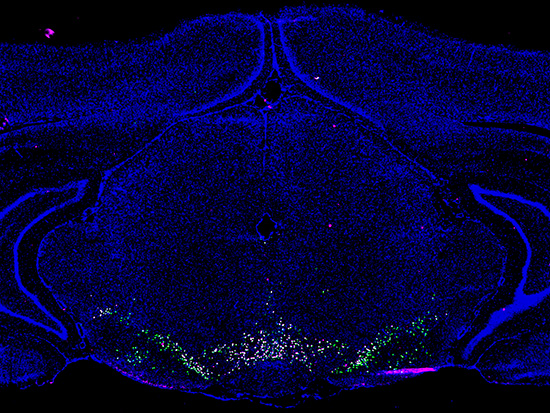
 Coronal section of rat brain marked for indicators of nuclei (DAPI, blue) or dopamine neurons (tyrosine hydroxylase, green; GTP cyclohydrolase 1, magenta).
Coronal section of rat brain marked for indicators of nuclei (DAPI, blue) or dopamine neurons (tyrosine hydroxylase, green; GTP cyclohydrolase 1, magenta).
Photo Credit: Emma AndrakaIn a work of systematic biology that advances the field, University of Alabama at Birmingham researchers have identified 16 distinct cell populations in a complex area of the midbrain called the ventral tegmental area, or VTA.
The VTA is important for its role in the dopamine neurotransmission involved in reward-directed behavior. Substance use disorders involve dysregulation of these reward circuits, leading to repeated drug-seeking despite adverse consequences. These include more than 100,000 drug overdose deaths in the United States in the most recent year. The VTA also has a role in several other neuropsychiatric disorders.
Thus, expanding knowledge of its function is a start to explaining the mechanisms for substance use disorders involving drugs like cocaine, alcohol, opioids and nicotine, or psychiatric disorders like schizophrenia and attention deficit hyperactivity, or ADHD.
Dopamine is one of the neurotransmitters used by the brain as chemical messengers to send signals between nerve cells. While decades of research have focused on dopaminergic neurotransmission in the VTA, there is also substantial evidence for the importance of two other neurotransmitters acting in the VTA in reward-related behaviors — GABA and glutamate. There is also evidence for “combinatorial” neurons that can potentially synthesize and release multiple neurotransmitters. These suggest an additional layer of complexity in VTA cellular and synaptic function.
Systematic biology is the science of classification, and it usually refers to the classification of organisms with regard to their natural relationships. The UAB VTA study classifies cell populations to extend and deepen previous work on the different cell types in the VTA, to provide a starting point for deciphering the relationships among these cells and their broad connections to other areas of the brain. The research, published in Cell Reports, was led by co-first authors Robert A. Phillips III and Jennifer J. Tuscher, Ph.D., and corresponding author Jeremy J. Day, Ph.D.
The 16 distinct cell populations were identified by differences in gene expression after single-nucleus RNA sequencing of 21,600 cells from the rat VTA, creating a searchable online atlas of the VTA. The rat is the prime model for reward and substance use studies. This unbiased approach — in contrast to previous studies that selected some subsets of cells for RNA sequencing — was used to create the largest and most comprehensive single-cell transcriptomic analysis focused exclusively on the composition and molecular architecture of the VTA.
Though it was well known that the VTA is composed of heterogeneous cell types, the UAB atlas expands those studies in several key ways.
 Jeremy Day, Ph.D.“For example, previous single-cell sequencing studies were conducted exclusively in the mouse brain and have relied primarily on sequencing a subset of fluorescence-activated cell sorting-isolated midbrain dopaminergic populations, rather than sampling all VTA cell types,” Day said. “Notably, our sequencing dataset focuses exclusively on VTA sub-regions, unlike other studies that have focused on pooled cells from the mouse substantia nigra and VTA or a subset of fluorescently tagged cells from general midbrain regions.”
Jeremy Day, Ph.D.“For example, previous single-cell sequencing studies were conducted exclusively in the mouse brain and have relied primarily on sequencing a subset of fluorescence-activated cell sorting-isolated midbrain dopaminergic populations, rather than sampling all VTA cell types,” Day said. “Notably, our sequencing dataset focuses exclusively on VTA sub-regions, unlike other studies that have focused on pooled cells from the mouse substantia nigra and VTA or a subset of fluorescently tagged cells from general midbrain regions.”
The 16 distinct cell populations include classic dopaminergic neurons, three subsets of glutamatergic neurons and three subsets of GABAergic neurons, as well as nine other cell types, including astrocytes and glial cells.
After sub-clustering neuronal cells, the UAB researchers also identified four sub-clusters that may represent neurons capable of combinatorial neurotransmitter release. They also identified selective gene markers for classically defined dopamine neurons and for the combinatorial neurons. A selective marker allows viral targeting of distinct VTA subclasses for functional studies.
The researchers also examined sub-clusters for opioid neuropeptides and their receptors, and identified pan-neuronal increased expression for risk genes associated with schizophrenia and “smoking initiation,” as well as enrichment of ADHD risk genes in two glutamatergic neuronal populations.
Co-authors besides Day, Phillips and Tuscher for the study, “An atlas of transcriptionally defined cell populations in the rat ventral tegmental area,” are Samantha L. Black, Emma Andraka and N. Dalton Fitzgerald, UAB Department of Neurobiology and Evelyn F. McKnight Brain Institute; and Lara Ianov, Civitan International Research Center at UAB. Day, Phillips and Tuscher are, respectively, associate professor, graduate student and postdoctoral fellow in the UAB Department of Neurobiology.
Support came from National Institutes of Health grants MH114990, DA039650 and DA048348; UAB’s Pittman Scholars Program, AMC21 Scholars Program and Civitan International Research Center; and a Brain and Behavior Research Foundation Young Investigator grant.
The UAB Department of Neurobiology, McKnight Brain Institute and Civitan International Research Center are all part of the Marnix E. Heersink School of Medicine.
